2. Acetone, wax, and lighter fluid. They're insoluble in water.
3. Ethanol is less polar than water.
4. Yes, I would expect ethanol to dissolve in water. I don't think hexane would dissolve in water.
5. NH3- Polar, Dissolves in water, Doesn't dissolve in hexane
I2- Non polar, Doesn't dissolve in water, Dissolves in hexane
HCL- Polar, Dissolves in water, Doesn't dissolve in hexane
6. Because water is polar, polar molecules will dissolve in it. Hexane is non polar, so they won't dissolve in it.
Enrichment: When CO2 dissolves into water, it happens slowly on the surface of the water. The CO2 crosses from the air into the liquid. It then reacts with the water. CO2 + H2O
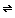 H2CO3 If this reaction didn't happen, all aquatic life would die because fish and plants underwater take the O2 and CO2 out of the H2O.
H2CO3 If this reaction didn't happen, all aquatic life would die because fish and plants underwater take the O2 and CO2 out of the H2O. 10.2 Section
The temperature made a big difference in how fast the KCIO3 dissolved. In order to make it dissolve faster, you need to increase the temperature. Doing this makes particles have less attraction to each other, move faster, and have more effective collisions. Unsaturated = Little to no solute. Saturated = Max amount of solute. Super Saturated = More than the max amount of solute. To make rock candy, boil water and sugar in a pan. Mix them until the sugar is dissolved in the water, making a saturated solution. When cooled, the solution turns super saturated. This is what forms the crystalline structure you see in rock candy.
No comments:
Post a Comment